Effect of Collagen Fiber Tortuosity Distribution on the Mechanical Response of Arterial Tissues
Abstract
This study investigated the effect of collagen fiber tortuosity distribution on the biomechanical failure and prefailure properties of arterial wall tissue. An in-silico model of the arterial wall was developed using data obtained from combined multiphoton microscopy imaging and uni-axial tensile testing. Layer-dependent properties were prescribed for collagen, elastin, and ground substance. Collagen fibers were modeled as discrete anisotropic elements, while elastin and ground substance were modeled as homogeneous isotropic components. Our parametric analysis, using a finite element approach, revealed that different parameters of collagen fibers tortuosity distribution significantly influence both prefailure and failure biomechanical properties. Increased fiber tortuosity improved the tissue strength whereas the dispersion in the tortuosity distribution reduced it. This study provides novel insights into the structural-mechanical interdependencies in arterial walls, offering potential targets for clinical assessments and therapeutic interventions aimed at mitigating rupture risks.
Methodology
We employed a multi-scale computational approach combining:
- Microstructural analysis of collagen fiber organization
- Statistical characterization of tortuosity distributions
- Finite element modeling of tissue mechanics
- Validation against experimental data
The following figure illustrates the computational model and results from our study on collagen fiber tortuosity distribution and its effect on arterial tissue mechanics.
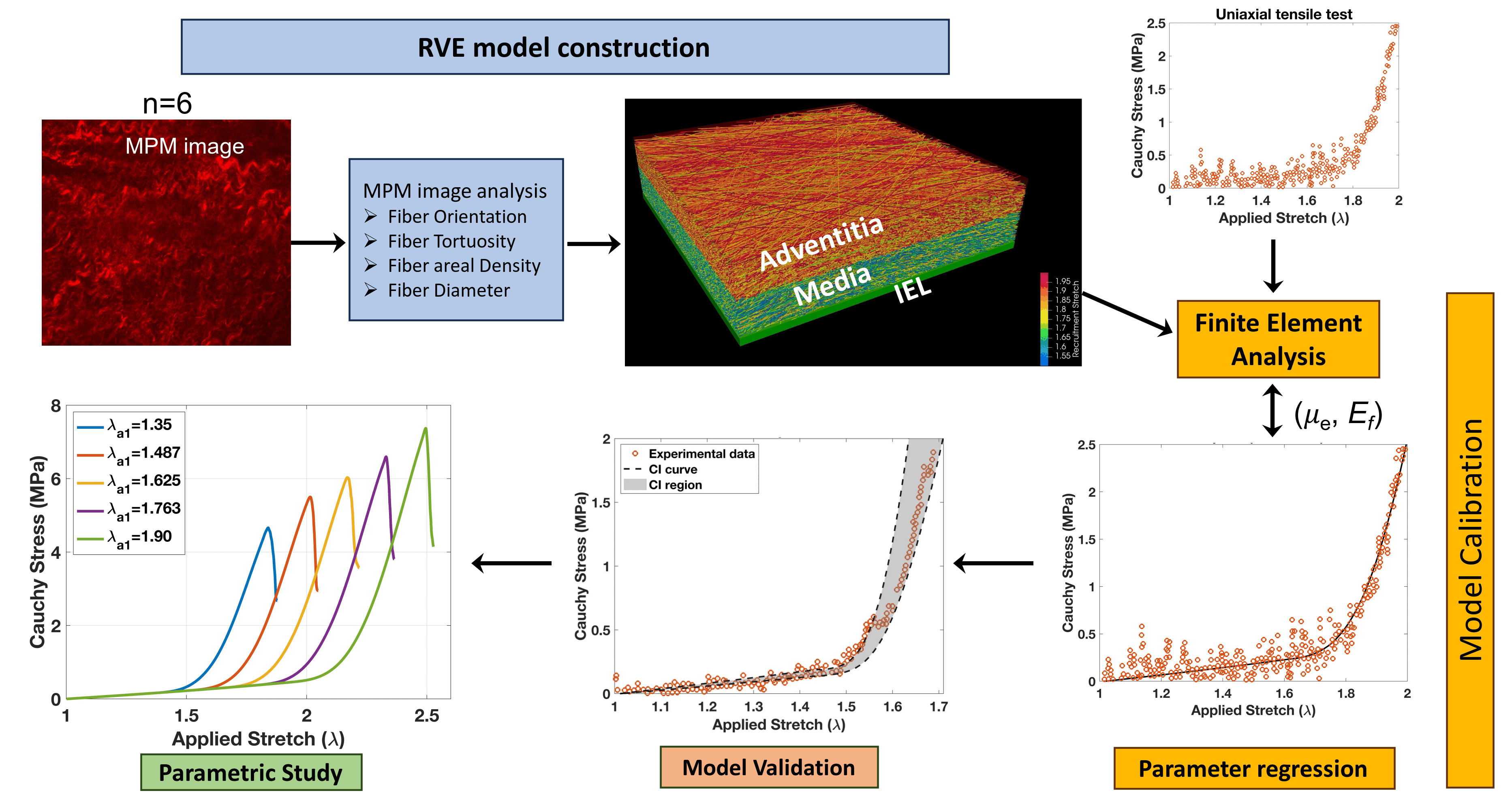
Figure: The computational workflow for representative arterial wall tissue model construction, model properties calibration using experimental tensile test data, model validation using confidence interval (CI) values, and parametric study on tortuosity distribution parameters. The tissue representative volume element (RVE) consists of three layers (i.e., internal elastic lamina (IEL), media, and adventitia). Fiber and matrix composition are also layer specific.
3D Interactive Model
Explore the 3D computational model used in this study. You can rotate, zoom, and pan to examine the tissue structure and stress distribution in detail.
Interactive 3D Visualization
3D model showing the arterial tissue representative volume element (RVE) with stress distribution. Use mouse to rotate (left click + drag), zoom (scroll), and pan (right click + drag). Click "Show Deformed" to visualize the mesh with displacement applied (warp by vector).
Constitutive Models
The arterial wall tissue is modeled as a composite material consisting of a soft matrix reinforced with collagen fibers. The constitutive behavior is described using hyperelastic models for both components.
-
Matrix Constitutive Model
The isotropic matrix (elastin and ground substance) is modeled using the Neo-Hookean hyperelastic model:
Where:
$\psi_{matrix}$ is the strain energy density function for the matrix, $\mu$ is the shear modulus of the matrix material, $K$ is the penalty parameter that enforces the incompressibility condition, $I_1$ is the first invariant of the right Cauchy-Green deformation tensor, $J$ is the determinant of the deformation gradient (volume ratio).
-
Collagen Fiber Constitutive Model
Individual collagen fibers are modeled using a piecewise linear constitutive model that accounts for the crimp state of fibers at low stretches:
Where:
$P_f$ = first Piola-Kirchhoff stress in the fiber direction, $E_f$ = elastic modulus of the collagen fiber, $\lambda_f$ = current fiber stretch, $\lambda_t$ = true fiber stretch (tortuosity adjusted), $\lambda_a$ = activation stretch (threshold for fiber engagement), $S$ = damage parameter of the collagen fiber.
This piecewise model captures the characteristic behavior of collagen fibers where they carry no load when they are not recruited ($λ_f$ < $λ_a$) and exhibit linear elastic behavior once engaged ($λ_f$ ≥ $λ_a$).
Results
The computational analysis revealed significant insights into how collagen fiber tortuosity affects the mechanical behavior of arterial tissues. The following animations demonstrate the stress-strain relationships and stress distributions observed in our simulations.
Note: Click any animation to restart all animations simultaneously for better comparison.
Stress-Stretch Curve Evolution
Animation illustrating the mechanical response curves showing how different tortuosity distributions affect the stress-stretch relationship of arterial tissue during loading.

Matrix Stress Evolution
Animation showing the evolution of stress patterns in the matrix component of arterial tissue during mechanical loading, demonstrating how tortuosity affects stress transfer mechanisms.
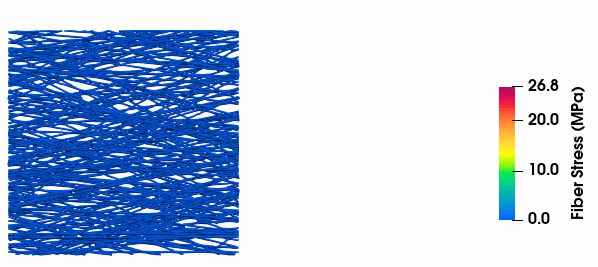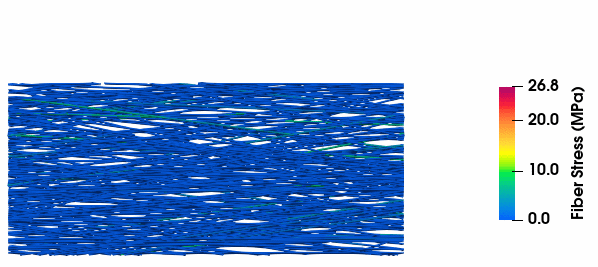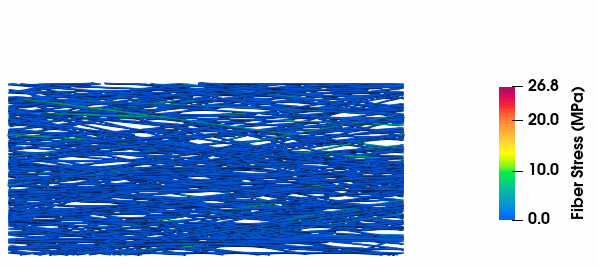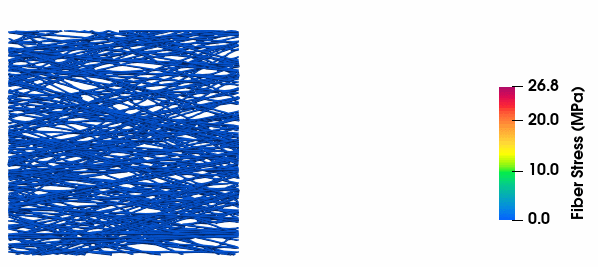
Fiber Stress Evolution
Visualization of stress development in collagen fibers with varying tortuosity, illustrating the relationship between fiber geometry and mechanical response.
Fiber Damage Evolution
Progressive damage accumulation in collagen fibers showing how tortuosity distribution influences failure patterns and tissue strength.
Key Findings
- Collagen fiber tortuosity significantly affects the mechanical response of arterial tissues
- Variations in tortuosity distribution lead to different stress-strain behaviors
- The developed computational model provides insights into tissue mechanics
- Results have implications for cardiovascular disease research
Conclusions
This work provides new insights into the relationship between collagen fiber microstructure and arterial tissue mechanics. The findings contribute to our understanding of how structural variations at the fiber level influence the overall mechanical behavior of arterial walls. This knowledge is essential for developing better models of cardiovascular disease progression and designing more effective treatment strategies.
The computational framework developed in this study can be extended to investigate other aspects of tissue mechanics and has potential applications in personalized medicine approaches for cardiovascular care.
Research Impact
Citations
Available on Google Scholar
Field
Biomechanics, Cardiovascular Engineering
Applications
Medical Device Design, Disease Modeling